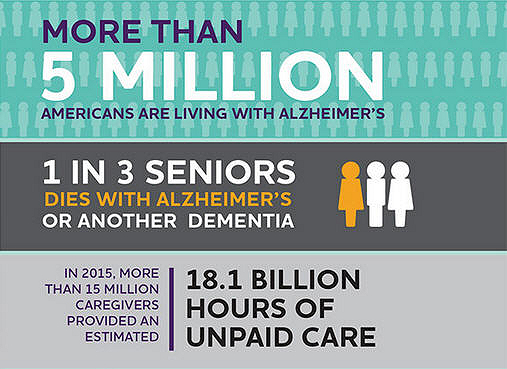
(Today.com) Alzheimer’s disease affects more than 5 million Americans — and according to the Alzheimer’s Association, every 66 seconds someone in the U.S. develops the disease. Today, there is hope for people with Alzheimer’s and those at risk: A new drug aims to stop the disease before symptoms start.
Harvard neurologist and Alzheimer’s researcher at Brigham and Women’s Hospital in Boston, Massachusetts, Dr. Reisa Sperling, is leading the new drug trial, and believes it will be a game changer.
“We’re really trying to detect Alzheimer’s disease before symptoms (start) and try to prevent people from developing the devastating memory loss,” Sperling told TODAY.
The 3 1/2-year study will monitor patients’ cognitive skills and brains to see if the drug has an impact, compared to people taking a placebo.
So how does it work? Alzheimer’s is associated with heavy protein buildup in the brain, referred to as amyloid. This buildup is linked to cognitive decline and memory loss. The new drug, solanezumab, is designed to stop the amyloid buildup in patients who experience early warning signs. It’s currently being tested in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (A4 study, for short).
“We want to help people live their lives with dignity and end their lives ballroom dancing, instead of in nursing homes,” Sperling said.
Research suggests that lifestyle factors can also help to boost your brain. Dr. David Perlmutter recommends steering clear of sugar and carbs, which can lead to the production of free radicals, which in turn may cause brain deterioration. Instead, he advises to follow a diet high in antioxidants, prebiotic fiber and healthy fats.
“It’s never too late to change your diet and begin some aerobic exercise,” Perlmutter told TODAY. “Research demonstrates that even in our 70s, even in our 80s, we have the ability to increase the growth of new brain cells.”
Though it’s important to note that these lifestyle changes are not a cure to the disease.
The A4 Study
The A4 Study is a landmark public-private partnership, funded by the National Institutes of Health, Eli Lilly and Company, and several philanthropic organizations. It is coordinated by the Alzheimer’s Therapeutic Research Institute, located at the University of Southern California.
See a list of study sites.
Research is taking place at 56 U.S. sites, as well as four sites in Canada and one in Australia.
You may qualify for the A4 study if you:
- Are 65 to 85 years old
- Have normal thinking and memory abilities
- Have elevated levels of amyloid in the brain, as shown by a PET brain scan
- Have a family member, friend, or other person with whom you have contact at least once a week who can answer questions about you once a year (contact may be in person or by phone)
- Are willing and able to receive IV infusions of the investigational treatment (solanezumab) or a placebo every 4 weeks for 3 years
- Are willing to have your health monitored using:
- memory and thinking tests
- electrocardiograms to look at your heart
- PET scan to look for amyloid plaques associated with Alzheimer’s
- MRI scans, another type of brain scan
- blood and urine tests
People who do not qualify for the main A4 study may be asked to participate in a separate study, in which they will take memory tests every 6 months.
To find out more about A4:
- Contact a local study site.
- Visit http://a4study.org
- Call 1-844-247-8839 (1-844-A-4-STUDY)
- Email [email protected]
View a video with Dr. Sperling:
View the video with Spanish subtitles:

Today
http://www.today.com/health/new-alzheimer-s-disease-drug-a4-may-help-people-risk-t99566
By Gabrielle Frank, Today
Copyright NBCUniversal Media, LLC
The A4 Study
https://www.nia.nih.gov/alzheimers/a4-study